Warum sollten Sie uns wählen?
Vertrauen
Vertrauen ist einer unserer wichtigsten Werte. Deshalb legen wir großen Wert auf die Sicherheit Ihrer Daten und tauschen diese nur über sichere Server in Deutschland mit Ihnen aus.
Kundenzufriedenheit
Ihre Zufriedenheit ist unser höchstes Ziel in allen Projekten. Ein Projekt ist für uns nur dann erfolgreich, wenn Sie zufrieden sind!
Zuverlässigkeit
Müssen Sie kurzfristig ein kritisches Problem lösen? Gemeinsam können wir das schaffen - wenn nötig auch mit einer Nachtschicht. Denn wir sind immer für unsere Kunden da.
Vier Gründe für unsere Zusammenarbeit bei der IVDR Leistungsbewertung
- Unser Geschäftsführer Lukas Losigkeit begann bereits in 2017 mit der Erstellung eines Konzepts zur IVDR Leistungsbewertung.
- Unser Konzept wurde seitdem stetig weiterentwickelt.
- Ihr Projekterfolg wird durch unsere Experten gesichert.
- Ihre Zufriedenheit ist unser wichtigstes Ziel.
Unsere Experten erstellen für Sie den Leistungsbewertungsplan (PEP) und Bericht über die Leistungsbewertung (PER) für IVDs der Klassen A bis D.
Mit thinqbetter haben Sie einen Partner, der schnell, unkompliziert und zuverlässig Ihre IVD Leistungsbewertung als externer Dienstleister erstellt.

IVDR Leistungsbewertung: unsere Dienstleistungen
thinqbetter erstellt IVDR Leistungsbewertungen für Sie. Unsere Experten unterstützen Sie in allen Aspekten der Erstellung und Aktualisierung. Und zwar unabhängig davon, ob Ihr Produkt Klasse A, B, C oder D ist.
Mit unseren Leistungsbewertungen sind Sie optimal auf die IVDR vorbereitet!
Wir aktualisieren bestehende Leistungsbewertungen - Komplett oder in Teilen. Ganz nach Ihren Bedürfnissen.
Profitieren Sie von unserer Erfahrung. Wir teilen diese gerne mit Ihnen!
IVD Leistungsbewertung nach IVDD
Achtung: Die nachfolgende Darstellung bezieht sich auf Produkte, die in der Übergangszeit zwischen der IVDD und IVDR (nach IVDD-Zulassung) in Verkehr gebracht wurden. Viele Produkte müssen lediglich neu unter IVDR zugelassen werden!
Die IVD Richtlinie 98/79/EC stellt in Anhang I Anforderungen an die Leistung von In-vitro-Diagnostika. Produkte sollen so ausgelegt sein, dass sie für ihre Zweckbestimmung geeignet sind und Leistungsparameter eingehalten werden. In dem Zusammenhang zu nennende Parameter sind unter anderem die folgenden:
- analytische Sensitivität,
- diagnostische Sensitivität,
- analytische Spezifität,
- diagnostische Spezifität,
- Richtigkeit,
- Wiederholbarkeit,
- Reproduzierbarkeit,
- einschließlich der Beherrschung der bekannten Interferenzen und Nachweisgrenzen
Doch wie weist man das nach? Wie wurde eine Leistungsbewertung unter der IVDD durchgeführt? Die Norm EN 13612 gibt dabei ein wenig Hilfestellung. Sie beschreibt die Leistungsbewertung von In-vitro-Diagnostika anhand einer Leistungsbewertungsstudie.
Die Anforderungen der IVDD und der EN 13612 waren sehr allgemein und wurden entsprechend unterschiedlich interpretiert. Im Mai 2022 trat die IVD-Verordnung (IVDR) in Kraft. Dort werden mittlerweile wesentlich umfangreichere Anforderungen an die Leistungsbewertung gestellt. Da sämtliche IVDD-Zulassungen auslaufen, müssen Leistungsbewertungen für zu überführende IVDs oder Neuzulassungen somit die Anforderungen der IVDR erfüllen.
Weiterführende Informationen zur Leistungsbewertung von IVDs für Software:
Für Software, die als IVD gilt, gibt es derzeit nur eine MDCG-Leitlinie, welche von der offiziellen Website der Europäischen Union heruntergeladen werden kann.
Auf unserem Blog finden Sie darüber hinaus weitere Materialien zum Thema IVDR Leistungsbewertung unter:
Schon vor der Produktzulassung in den USA gibt es vieles zu beachten:
IVDR Leistungsbewertung – Was ändert sich in Zukunft?
Haben Sie schon einmal die MEDDEV 2.7-1 Revision 4 zur klinischen Bewertung von Medizinprodukten gelesen? Sind Sie der Meinung, dass das ein sehr umfangreiches, sehr formales Verfahren ist? Die Leistungsbewertung nach IVDR entwickelt sich in eben diese Richtung.
Die bisher sehr unterschiedlich gehandhabten Leistungsbewertungen für In-vitro-Diagnostika werden unter der IVDR einem einheitlicheren Verfahren unterzogen. Der Artikel 56 und Anhang XIII geben eine ganz neue Fülle an Anforderungen zur Leistungsbewertung.
Wie auch bei der klinischen Bewertung gemäß MDR, wird die Leistungsbewertung gemäß IVDR zweigeteilt:
- Ein Leistungsbewertungsplan wird gefordert. Die notwendigen Inhalte sind in Anhang XIII Absatz 1.1 aufgeführt.
- Der Leistungsbewertungsbericht bzw. Bericht über die Leistungsbewertung beinhaltet mindestens die Inhalte aus Anhang XIII, Absatz 1.3.2.
Der Bericht über die Leistungsbewertung soll die zur Literaturrecherche verwendete Methodik, das Literatursuchprotokoll und einen Bericht zur Literaturauswertung enthalten. Wie so eine Literaturrecherche durchgeführt und dokumentiert wird, hat sich bereits im Bereich der Medizinprodukte und der MEDDEV 2.7-1 Revision 4 etabliert.
Das Prinzip der IVDR Leistungsbewertung
Eine IVDR-konforme Leistungsbewertung folgt einem methodologischen Prinzip. Sie ist gründlich, objektiv und berücksichtigt günstige sowie ungünstige Daten. Maßgebend für die Gründlichkeit und den Umfang ist das Produkt und die damit verbundene Risikoklasse, bzw. Risiken, Leistung und Zweckbestimmung.
Ein IVD Hersteller muss einschlägige verfügbare Daten für das Produkt und die Zweckbestimmung ermitteln. Sollten anschließend ungelöste Fragen oder Datenlücken vorhanden sein, ist eine systematische Auswertung wissenschaftlicher Literatur durchzuführen, um diese Fragen zu adressieren. Sollte dies nicht ausreichend möglich sein, sind entsprechende Daten zu erzeugen. Alle ermittelten Daten müssen für die Eignung zur Bestimmung der Sicherheit und Leistung bewertet werden.
Die IVDR Leistungsbewertung basiert auf 3 Säulen:
- Nachweis der wissenschaftlichen Validität
- Nachweis der Analyseleistung
- Nachweis der klinischen Leistung
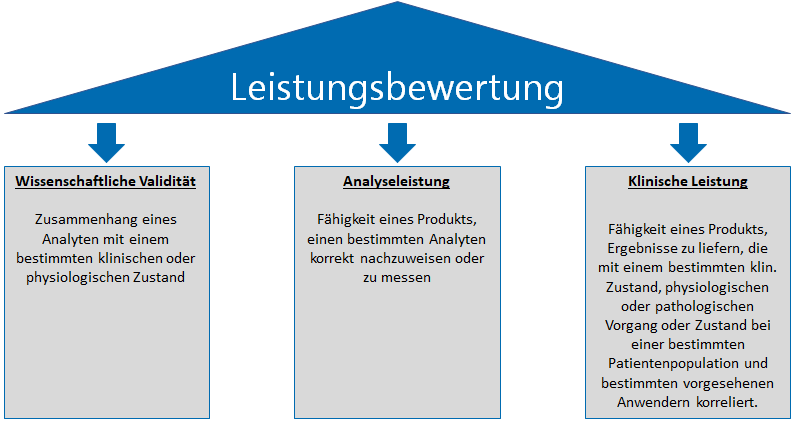
Die wissenschaftliche Validität eines Analyten „bezeichnet den Zusammenhang eines Analyten mit einem bestimmten klinischen oder physiologischen Zustand [IVDR]“. Der Weg über wissenschaftliche Literatur ist ein einfacher Weg, diesen Aspekt nachzuweisen. Sofern verfügbar, können andere Quellen wie z.B. Ergebnisse aus klinischen Leistungsstudien herangezogen werden.
Die Analyseleistung bezeichnet “die Fähigkeit eines Produkts, einen bestimmten Analyten korrekt nachzuweisen oder zu messen [IVDR]“. Hierfür sind grundsätzlich analytische Leistungsstudien durchzuführen. Welche Parameter nachzuweisen sind, geht aus Anhang I Abschnitt 9.1 Buchstabe a hervor. Dort werden unter anderem folgende Parameter beschrieben:
- analytische Sensitivität,
- analytische Spezifität,
- Richtigkeit (Verzerrung),
- Präzision (Wiederholbarkeit und Reproduzierbarkeit),
- usw.
Die klinische Leistung bezeichnet „die Fähigkeit eines Produkts, Ergebnisse zu liefern, die mit einem bestimmten klinischen Zustand oder physiologischen oder pathologischen Vorgang oder Zustand bei einer bestimmten Zielbevölkerung und bestimmten vorgesehenen Anwendern korrelieren [IVDR]“. In Anhang I Abschnitt 9.1 Buchstabe b werden relevante Parameter der klinischen Leistung wie folgt genannt:
- diagnostische Sensitivität,
- diagnostische Spezifität,
- positiver prädiktiver Wert, negativer prädiktiver Wert,
- Likelihood-Verhältnis
- und erwartete Werte bei nicht betroffenen und betroffenen Bevölkerungsgruppen
An dieser Stelle ist interessant, wie der Nachweis der klinischen Leistung erbracht werden kann. Es besteht die Möglichkeit, eine oder eine Kombination der folgenden Quellen dafür heranzuziehen:
- klinische Leistungsstudien
- wissenschaftliche Literatur
- veröffentlichte Erfahrungen aus diagnostischen Routinetests
Sofern ausreichende Gründe vorhanden sind, kann auf die Durchführung klinischer Leistungsstudien verzichtet werden. Hier wird deutlich, dass der Weg über klinische Leistungsstudien bevorzugt wird. Allerdings ist dies häufig nicht im Sinne der Hersteller. Der Ausweg: Die erforderlichen Gründe gegen eine Studie werden über wissenschaftliche Literatur dargelegt. Dies stärkt wiederum die Wichtigkeit der Literaturrecherche in der IVDR Leistungsbewertung. Die Ähnlichkeit zur klinischen Bewertung wird wieder deutlich.
Warum sollten Sie uns wählen?
Vertrauen
Vertrauen ist einer unserer wichtigsten Werte. Deshalb legen wir großen Wert auf die Sicherheit Ihrer Daten und tauschen diese nur über sichere Server in Deutschland mit Ihnen aus.
Kundenzufriedenheit
Ihre Zufriedenheit ist unser höchstes Ziel in allen Projekten. Ein Projekt ist für uns nur dann erfolgreich, wenn Sie zufrieden sind!
Zuverlässigkeit
Müssen Sie kurzfristig ein kritisches Problem lösen? Gemeinsam können wir das schaffen - wenn nötig auch mit einer Nachtschicht. Denn wir sind immer für unsere Kunden da.
Literaturrecherche zur IVDR Leistungsbewertung
- Schritt 1: Analysieren der Zweckbestimmung und klinischen Claims, inklusive der Patientenpopulation, Indikationen, Kontraindikationen. Zu den hier identifizierten Aspekten werden in der Literaturrecherche Daten ermittelt.
- Schritt 2: Identifikation von Datenquellen, aus denen klinische Daten gewonnen werden können. Beispielsweise PubMed, Cochrane, Embase.
- Schritt 3: Erstellen von Suchbegriffen und objektiven Filtern, um Daten über das zu bewertende In-vitro-Diagnostikum zu ermitteln.
- Schritt 4: Die identifizierte Literatur wird zunächst auf Ebene der Abstracts vorgefiltert und auf potenzielle Relevanz geprüft.
- Schritt 5: Potenziell relevante Literatur wird nun auf Ebene des Volltextes tiefgehend analysiert.
- Schritt 6: Die in der Analyse der Volltexte gewonnenen klinischen Leistungsdaten werden nun genutzt, um die klinische Leistung zu belegen und ggf. zu begründen, dass keine klinische Leistungsstudie durchgeführt werden muss.

Stand der Technik in der IVDR Leistungsbewertung
Der Leistungsbewertungsplan soll eine Beschreibung des neuesten Stands der Technik, einschließlich der Angabe einschlägiger Normen, gemeinsamer Spezifikationen, Leitlinien oder Dokumente über vorbildliche Verfahren enthalten. Zusätzlich wird die Angabe und Spezifizierung der Parameter zur auf dem neuesten medizinischen Kenntnisstand beruhenden Bestimmung der Annehmbarkeit des Nutzen-Risiko-Verhältnisses für die Zweckbestimmung und für die Analyse- und klinische Leistung des Produkts gefordert.
Es wird deutlich, dass das zu bewertende In-vitro-Diagnostikum nicht für sich allein betrachtet wird, sondern auch, wie sich das Produkt zum Stand der Technik und neusten medizinischen Kenntnisstand verhält.
Im Bericht über die IVDR Leistungsbewertung wird dann dargelegt, dass der Nachweis der klinischen Leistung vor dem Hintergrund des neusten medizinischen Kenntnisstands akzeptabel ist. Die Literaturrecherche ist eine Möglichkeit, um hierfür hilfreiche Informationen zu ermitteln.
IVDR Leistungsbewertung: Schnittstelle zur Überwachung nach dem Inverkehrbringen
Das System zur Überwachung nach dem Inverkehrbringen kann aktiv und systematisch Daten über die Qualität, Leistung und Sicherheit des IVDs während der gesamten Lebensdauer sammeln. Die hier gesammelten Informationen können zur Aktualisierung der Leistungsbewertung genutzt werden.
IVDR Leistungsbewertung: Schnittstelle zur Nachbeobachtung der Leistung nach dem Inverkehrbringen
Die Nachbeobachtung der Leistung unter IVDR ist ein fortlaufender Prozess, der die Leistungsbewertung aktualisiert. Dafür wird ein einzelner Plan benötigt, in dem die durchzuführenden Aktivitäten geplant werden. Die Inhalte dafür sind in Anhang XIII, Teil B, 5.2 beschrieben. Die IVDR verfolgt den Ansatz, dass einerseits während der gesamten Lebensdauer über das System zur Überwachung nach dem Inverkehrbringen Daten gesammelt, und über die Nachbeobachtung der Leistung dauerhaft Daten erzeugt werden.
Sofern eine Nachbeobachtung der Leistung nach dem Inverkehrbringen für ein IVD als nicht angemessen definiert, so wird eine Begründung dafür im Bericht über die Leistungsbewertung bereitgestellt. Ein Plan zur Nachbeobachtung der Leistung ist dennoch notwendig. Er wird jedoch auf die Begründung in der Leistungsbewertung verweisen.

Die wichtigsten Unterschiede zwischen IVDD und IVDR Leistungsbewertung
Ein wesentlicher Unterschied bezieht sich auf den Rechtscharakter der beiden Leistungsbewertungen:
- Die IVDR ist die aktuelle EU-Verordnung, die den Rechtsrahmen für das Inverkehrbringen von IVDs in der EU regelt. Sie ist auch als EU 2017/746 bekannt.
- Bei der IVDD handelt es sich indes um eine nicht länger gültige EU-Richtlinie, die früher den genannten Rechtsrahmen dargelegt hat. Die IVDD ist im englischsprachigen Raum auch als 98/79/EC bekannt, im deutschsprachigen als 98/79/EG.
Wichtig zu wissen: Eine EU-Verordnung hat Gesetzescharakter in den einzelnen EU-Mitgliedsstaaten, eine Richtlinie nicht.
Momentan befinden wir uns in einer Übergangsphase: Es gibt auf dem Markt nach wie vor viele Produkte, die auf den alten IVDD-Zulassungen basieren und zur IVDR überführt werden müssen, weil die alten Zulassungen auslaufen (zeitliche Begrenzung).
Zusammenfassend lässt sich konstatieren, dass die IVDR wesentlich umfangreichere Anforderungen stellt, die von IVDs eingehalten werden müssen. Insbesondere werden eine Vielzahl unterschiedlicher Leistungsmerkmale zur Analyseleistung und klinischen Leistung benötigt, die unter IVDD oft nicht erhoben wurden.
Schlussfolgerung
Die IVDR Leistungsbewertung ist wesentlich umfangreicher als zuvor unter der IVDD. Daraus resultiert ein großer Mehraufwand, eine IVDR-konforme technische Dokumentation zu erzeugen. Hinzu kommt, dass viele Produkte höher klassifiziert werden. Als Anhaltspunkt: ca. 80 % der Produkte benötigten unter der IVDD keine benannte Stelle. Die IVDR führt dazu, dass nur ca. 20% der Produkte keine benannte Stelle benötigen. Viele Hersteller erfahren dadurch eine Fülle an Anforderungen und Regulierung, die sie bisher nicht kannten. In vielen Fällen müssen umfangreiche Studien zur Analyseleistung und klinischen Leistung von bestehenden IVDs durchgeführt werden.
Deshalb ist thinqbetter der ideale Partner für die IVDR Leistungsbewertung
Dank unserer Expertise und langjährigen Erfahrung im Bereich der IVDR Leistungsbewertung können wir Sie rund um das Thema umfassend beraten und begleiten. Sie können jederzeit darauf vertrauen, dass wir individuelle pragmatische und effiziente Lösungen für Sie finden und die bestmögliche Projektstrategie entwickeln. Dabei fokussieren wir uns stets auf sichere, leistungsfähige und konforme IVDs. Bei Bedarf stellen wir gerne Kontakte zu Partnern aus unserem Netzwerk zur Durchführung von Leistungsstudien her. Wir arbeiten zuverlässig, seriös und genießen das Vertrauen einer treuen Stammkundschaft. Wir sind Ihr starker, kompetenter und vertrauenswürdiger Partner – als Berater oder Dienstleister.
Unsere Qualifikation
Wir durften bereits über 140 verschiedene Unternehmen betreuen und haben dabei jahrelange Erfahrung in vielfältigen Fachbereichen gesammelt. Daraus resultieren eine optimierte Vorgehensweise, kurze Projektlaufzeiten und eine gleichbleibend hohe Effizienz sowie Qualität.
Wir erfassen Projektaufwände kleinteilig, um Optimierungspotenziale zu identifizieren und Arbeitsabläufe kontinuierlich zu verbessern. Unser Team kommt aus naturwissenschaftlichen Disziplinen wie Medizintechnik, Biologie, Chemie, Biotechnologie und Immunologie und vereint Erfahrungen aus Forschung, Beratung und Herstellung.
Unsere Mitarbeiter erhalten regelmäßige Schulungen, damit sie bestmöglich für die anfallenden Aufgaben gerüstet sind. Zusätzlich setzen wir Projektmanagement-Tools und ein internes Qualitätsmanagementsystem ein, um Prozesse und Abläufe klar zu strukturieren und damit Effizienz und Qualität zu sichern oder weiter zu steigern.


Unser Leistungsumfang im Bereich IVD
Unsere Angebote sind für Hersteller von Medizinprodukten und Hersteller von In-vitro-Diagnostika (IVDs) im B2B-Bereich konzipiert. Dazu gehören im Bereich der IVDR Leistungsbewertung nicht nur ein kostenloses Erstgespräch, sondern auch folgende Punkte:
- Erstellung von Leistungsbewertungen für In-vitro-Diagnostika (IVDs)
- Aktualisierung von Leistungsbewertungen für In-vitro-Diagnostika (IVDs)
Zusätzlich bieten wir weitere Dienstleistungen sowie Beratungen zu regulatorischen Themen an.
Am besten nehmen Sie heute noch Kontakt zu uns auf und vereinbaren ein kostenloses Erstgespräch!
Häufig gestellte Fragen
Was ist eine IVDR Leistungsbewertung?
Eine IVDR Leistungsbewertung wird lt. IVDR wie folgt definiert: „Leistungsbewertung“ bezeichnet eine Beurteilung und Analyse von Daten zur Feststellung oder Überprüfung der wissenschaftlichen Validität, der Analyseleistung und gegebenenfalls der klinischen Leistung eines Produkts. IVDR bezieht sich auf die EU 2017/746 und steht für „In vitro diagnostic regulation“.
Wann muss die IVDR Leistungsbewertung erstellt werden?
Bei Neuentwicklungen sollte die IVDR Leistungsbewertung frühzeitig begonnen werden, um über den Stand der Technik notwendige Richtwerte zur Sicherheit und Leistung zu ermitteln. So wird sichergestellt, dass das Produkt am Ende der Entwicklung auch dem Stand der Technik entspricht. Bei Überführungen von der IVDD zur IVDR sollte so früh wie möglich begonnen werden, weil häufig festgestellt wird, dass es erhebliche Datenlücken in den Bereichen Analyseleistung und/oder klinischer Leistung gibt.
Wie kann man sich auf die IVDR Leistungsbewertung am besten vorbereiten?
Wir bieten hierfür Schulungen und Workshops an und unterstützen Sie ganz individuell bei der Vorbereitung. Unabhängig davon ist es wichtig, sich mit Gap Analysen auf die IVDR Leistungsbewertung vorzubereiten. Damit erkennen Sie schnell, welche Datenlücken es in den Bereichen der Analyseleistung und/oder klinischen Leistung gibt.
Infos
anfordern
Einer unserer Berater wird Sie kurz nach Erhalt Ihrer Anfrage kontaktieren.
Vertraulichkeit und Diskretion sind für uns wichtig. Daher werden wir Ihre Anfrage entsprechend behandeln, auch bevor wir eine Vertraulichkeitsvereinbarung abschließen.
