Why choose us?
Trust
Trust is one of our most important values. That is why we attach great importance to the security of your data and exchange them with you only via secure servers in Germany.
Customer satisfaction
Your satisfaction is our highest goal in all projects. A project is only successful for us if you are satisfied!
Reliability
Do you have to solve a critical issue at short notice? Together we can do this - if necessary, even with a night shift. Because we are always available for our customers.
Four reasons for our cooperation
- Our experts provide your employees with a secure framework to achieve top performance.
- We prepare you optimally for the MDSAP audit.
- Your project success is ensured by our experts.
- Your satisfaction is our most important goal.
Our experts support you in all matters related to the Medical Device Single Audit Program.
With thinqbetter you have the right partner to successfully complete the MDSAP audit.

Our Services
thinqbetter is your partner for professional services in the field of MDSAP and quality management.
Whether training, gap analysis or support in adapting processes: we are there for you!
Our experts will also accompany you through the audit if you wish.
Deviations occur, especially in the initial MDSAP audit. We close these with you.
What is MDSAP?
MDSAP stands for “Medical Device Single Audit Program” – in other words, a program to check the country-specific requirements of the members. There are currently 5 participating regulatory authorities.
There is a so called Companion Document, which lists the MDSAP requirements. These are sorted according to the main processes that MDSAP identifies:
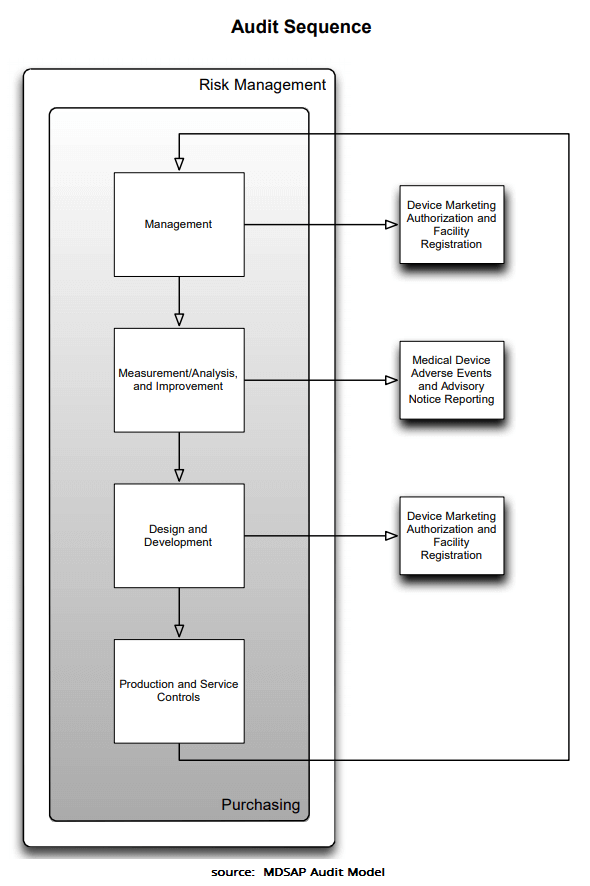
- Chapter 1 Process: Management
- Chapter 2 Process: Device Marketing Authorization and Facility Registration
- Chapter 3 Process: Measurement, Analysis and Improvement
- Chapter 4 Process: Medical Device Adverse Events and Advisory Notices Reporting
- Chapter 5 Process: Design and Development
- Chapter 6 Process: Production and Service Controls
- Chapter 7 Process: Purchasing
The Companion Document not only describes the individual requirements, it also contains explanations and links between the individual tasks. This gives a good overview of what is verified and expected in the MDSAP audit.
How does an MDSAP audit work?
This is described in great detail in the Audit Model. The initial Audit is split into two stages:
Stage 1 includes a remote quality management system audit. At this point it is verified whether the company or the QMS is ready for Stage 2. If non-conformities occur, these are communicated by the auditing organisation before the start of Stage 2.
Stage 2 is the audit on site at the manufacturer’s premises, in which a more in-depth check is made as to whether all requirements are met.
In the audit, the “audit sequence” is followed. This is shown in the graphic below. It clearly shows how much focus is placed on risk management and procurement.
Which MDSAP members are there?
- ANVISA – Brazil’s Agência Nacional de Vigilância Sanitária
- FDA – U.S. Food and Drug Administration
- HC – Health Canada
- MHLW – Japan’s Ministry of Health, Labour and Welfare, and the Japanese Pharmaceuticals and Medical Devices Agency
- TGA – Therapeutic Goods Administration of Australia
In addition to the listed “full” members, there are also the so-called “affiliate members”.
What is a MDSAP affiliate member?
The definition of the Affiliate Member describes the following: “A non-participating MDSAP Observer or non-participating MDSAP RAC regulatory authority that wants to engage in MDSAP, demonstrates understanding of MDSAP and utilize MDSAP audit reports and/or MDSAP certificates for evaluating a medical device manufacturer’s quality management system.”
The abbreviation RAC stands for “Regulatory Authority Council”. The RAC consists of representatives from all MDSAP members (Australia, Brazil, Japan, Canada, USA) and provides guidance, oversight and resources to support the development, implementation, maintenance and enhancement of the MDSAP.
What is the European Union’s position on MDSAP?
The EU is an “MDSAP Official Observer”. This describes regulatory authorities or potential members who may decide to participate in MDSAP and become active members. Basically, the introduction of the MDR and IVDR lays the foundation for the EU to participate in MDSAP. Previously, all specific country requirements of EU members must have been covered by MDSAP. Understandably, this would have resulted in an enormous effort to comply with the EU requirements. Now, the MDR and IVDR apply uniform requirements in Europe and thus provide a realistic basis for MDSAP participation.
As observer the EU can participate in meetings, evaluations and other activities of the MDSAP meetings.

Further information
If you are looking for further information about the topic, feel free to read our MDSAP blog post or just contact us. We are looking forward to hearing from you!
Why Choose Us?
Trust
Trust is one of our most important values. That's why we place great importance on the security of your data and only exchange it with you via secure servers in Germany.
Customer Satisfaction
Your satisfaction is our highest goal in all projects. For us, a project is only successful if you are satisfied!
Reliability
Do you need to solve a critical problem at short notice? Together, we can do it – even with an overnight shift if necessary. Because we are always there for our customers.
Request info
One of our consultants will contact you shortly after receiving your inquiry.
Confidentiality and discretion are important to us. Therefore, we will treat your inquiry accordingly, even before we conclude a confidentiality agreement.
